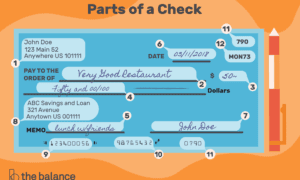
In the complex world of pharmaceutical development, clinical trials represent both a critical opportunity and a significant challenge. The site selection process, crucial for trial success, has traditionally relied on experience-based decisions, leading to inefficiencies, delays, and escalating costs. However, a groundbreaking integration of Artificial Intelligence (AI), Large Language Models (LLMs), and Master Data Management (MDM) is reshaping this process. This transformative approach, discussed by Sumit Prakash Singh in his recent article, promises not only to streamline site selection but also to significantly enhance the speed and accuracy of clinical trials.
The Strain of Traditional Site Selection
Clinical trials are essential for the development of new pharmaceuticals, but the process of selecting the right trial sites has long been plagued by inefficiency. Traditional methods of site selection often rely on outdated data, subjective decision-making, and manual processes, all of which contribute to delays. Research has shown that ineffective site selection can account for up to 30% of trial delays. The increasing complexity of trial protocols and the need for specific patient populations only exacerbate these challenges. As the pharmaceutical industry faces growing pressures to expedite drug development, the inefficiencies in site selection have become a major bottleneck.
Harnessing the Power of AI and LLMs for Smarter Site Selection
AI and Large Language Models (LLMs) revolutionize site selection by analyzing vast historical data to predict site performance with high accuracy. Unlike traditional methods, AI evaluates multiple performance factors, such as patient recruitment efficiency, protocol adherence, and data quality, enabling data-driven decisions that reduce trial delays and enhance success rates. LLMs further improve this process by assessing unstructured data, like investigator publications and trial history, to evaluate capabilities. Combined, AI and LLMs match site demographics with trial requirements, ensuring optimal site selection and avoiding costly trial and error.
Master Data Management: The Backbone of Predictive Analytics
Master Data Management (MDM) is essential for enabling predictive analytics in the pharmaceutical industry by integrating and harmonizing disparate datasets. MDM systems break down data silos, providing real-time tracking and more precise decision-making. By creating a unified data repository, MDM ensures a single source of truth, giving AI algorithms access to accurate and comprehensive data. Additionally, MDM enhances data governance, maintaining high-quality, standardized data across the organization. This improves AI model performance and boosts the reliability of predictive analytics. The integration of MDM with AI and LLMs streamlines the site selection process, reducing the time and resources spent on data reconciliation, thus accelerating trial initiation and improving overall efficiency.
Transforming Clinical Trials: Benefits Beyond Site Selection
The integration of AI, LLMs, and MDM in clinical trials offers numerous benefits, notably reducing site activation timelines from up to 20 weeks to just 8-12 weeks. This accelerated process speeds up drug development, giving pharmaceutical companies a competitive advantage. AI-driven systems also enhance site performance forecasting, optimizing resource allocation and cutting unnecessary costs. Predictive models identify potential risks early, allowing proactive interventions to prevent delays and minimize protocol deviations, resulting in significant cost savings and smoother trial execution.
A Clear Path to Implementation
The article offers a detailed roadmap for the successful implementation of these technologies. By adopting a phased approach, organizations can build a solid foundation of high-quality data, develop AI models for predictive analytics, and integrate these capabilities into daily workflows. This structured implementation ensures that pharmaceutical companies can realize the full benefits of AI and MDM without disrupting their ongoing operations.
In conclusion, as the pharmaceutical industry continues to seek ways to streamline operations and reduce costs, the integration of AI, LLMs, and MDM stands out as a transformative solution. By moving from traditional, experience-based site selection to a data-driven, predictive approach, clinical trials can be conducted more efficiently, with improved outcomes and lower costs. The adoption of these technologies is not just a technological shift but a strategic advantage, positioning pharmaceutical companies to accelerate the development of life-saving treatments. As highlighted by Sumit Prakash Singh, the future of clinical trials is here, and it’s powered by data, intelligence, and precision.