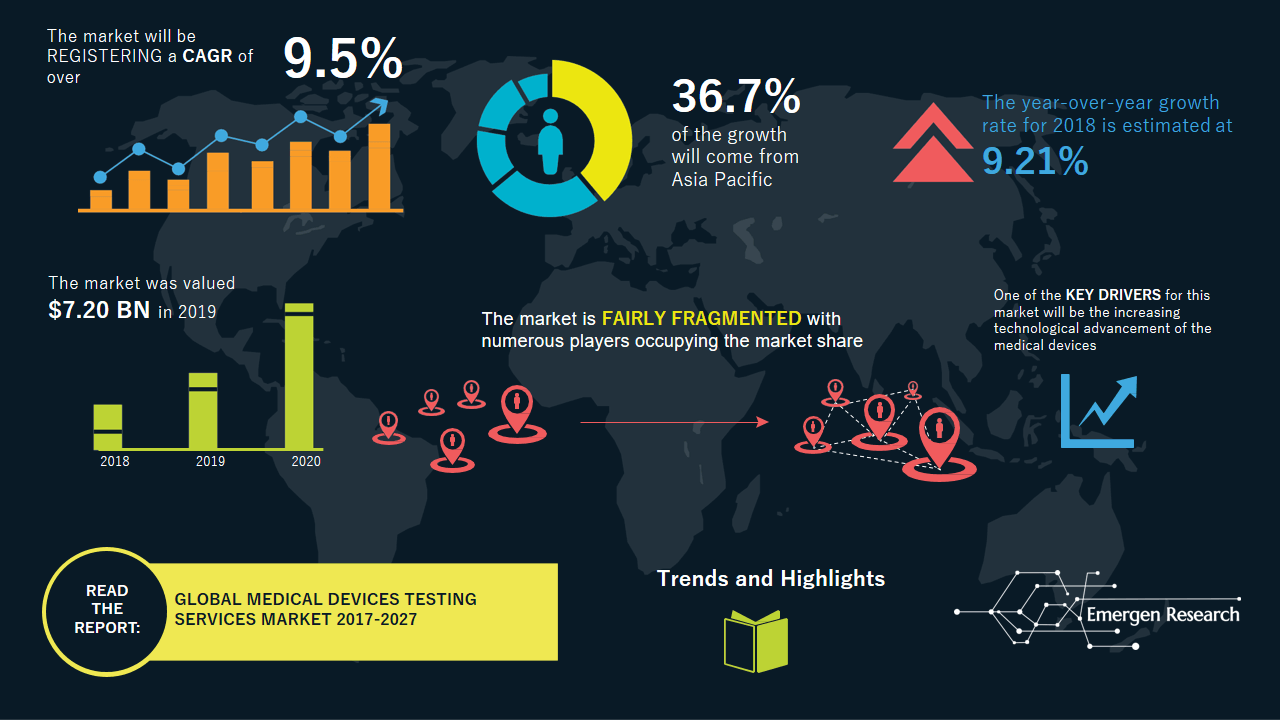
The medical device testing services market is expected to be valued at USD 14.25 billion in 2027 from USD 7.20 billion in 2019, registering a CAGR of 9.5% through the forecast period according to a recent report by Emergen Research. The expansion of this market can be attributed to the increasing demand for medical device testing. The increasing need for improved accuracy and durability of medical devices has resulted in the increasing adoption by pharmaceutical companies of testing services. Medical device manufacturers outsource testing services to third-party entities in order to reduce the increasing recalls of products, which is expected to drive the growth of the market for medical devices testing services. It is most likely that the presence of stringent regulatory protocols to ensure the safety of devices will fuel the demand for medical device testing services. The increasing demand for in-vitro tests to detect infectious diseases and monitor drug therapies and patients’ overall health status is a well-observed trend in the market for medical devices testing services.
Grab Your Free Sample PDF Copy Now
Key Highlights From The Report
- Thomas J. Stephens & Associates, Inc., a nationally recognised clinical research organisation serving the cosmetic and personal care industry, was acquired by SGS in January 2020. The acquisition is expected to expand SGS’s consumer & retail service portfolio in the cosmetic and personal care products clinical testing sector in the USA.
- In 2019, the preclinical sub-segment held the largest market share of 57.6%. The growth of the segment is expected to be driven by increasing investments in the R&D of preclinical medical device testing.
- Microbiology & sterility testing over the forecast period is projected to grow with the fastest CAGR of 10%. The growth of the segment is expected to be driven by increased investments from the biotechnology and pharmaceutical industries in testing services and increased research.
- Over the forecast period, the Asia Pacific region is expected to be the fastest growing market for medical devices testing services. The region’s medical devices testing services market share is driven by increasing government initiatives to improve the healthcare infrastructure and the availability of skilled labour at lower costs.
- Key participants include SGS S.A., Toxikon, Inc., Intertek Group plc, Pace Analytical Services, Charles River Laboratories International, Inc., American Preclinical Services LLC, North American Science Associates, Inc., Sterigenics International LLC, Eurofins, and WuXi AppTec Group, among others.
Emergen Research has segmented the global Medical Devices Testing Services Market on the basis of phase, service, and region:
- Phase Outlook (Revenue, USD Billion; 2017-2027)
- Clinical
- Preclinical (Medical Coatings and Antimicrobial Wound Dressings)
- Service Outlook (Revenue, USD Billion; 2017-2027)
- Chemistry Test
- Package Validation
- Microbiology & Sterility Testing (Pyrogen and Endotoxin Testing, Anti-microbial Activity Testing, Sterility Test and Validation and Bio-Burden Determination)
- Biocompatibility Tests
- Regional Outlook (Revenue, USD Billion; 2017-2027)
- North America
- U.S.
- Canada
- Europe
- Germany
- U.K.
- France
- BENELUX
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- Rest of APAC
- Latin America
- Brazil
- Rest of LATAM
- Middle East & Africa
- Saudi Arabia
- U.A.E.
- Rest of MEA
- North America
To get leading market solutions, visit the link below: https://www.emergenresearch.com/industry-report/medical-devices-testing-services-market