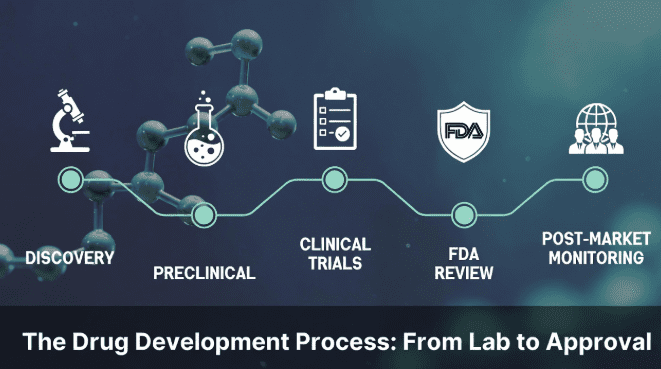
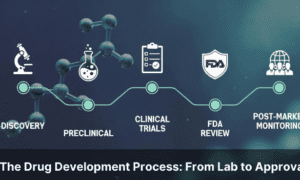
The drug development process is a structured sequence of stages that takes a compound from initial laboratory discovery through preclinical testing, clinical trials, regulatory review, and ultimately to market approval. On average, this journey takes 10 to 15 years and costs over $2 billion, with fewer than 12% of candidates that enter clinical trials ever reaching patients.
Understanding how drugs are developed matters whether you’re a researcher, a student, or simply someone trying to make sense of headlines about new treatments. Each phase of drug development serves a specific purpose—filtering out compounds that don’t work, confirming safety in those that do, and building the evidence regulators need before approving a new therapy.
This guide walks through the five core stages of drug development, explains what happens at each step, and uses peptide-based therapies as a running example to show how the process works in practice today.
Stage 1: Discovery and Development
Every drug begins with a question: can we find a compound that interacts with a specific biological target involved in disease?
During the discovery phase, researchers identify potential drug targets—usually proteins, receptors, or enzymes linked to a particular condition. They then screen thousands of compounds to find “hits” that interact with these targets. These hits are refined into “lead compounds” through a process called lead optimization, where scientists adjust the molecule’s structure to improve its potency, selectivity, and stability.
For peptide-based drug candidates, discovery often starts with naturally occurring peptides the body already produces. GLP-1 (glucagon-like peptide-1), for example, is a gut hormone involved in blood sugar regulation. Researchers recognized its biological activity decades ago, but the natural version degrades within minutes. The drug discovery process involved engineering modified versions that remain active for hours or days—a breakthrough that eventually led to an entire class of metabolic therapies.
This stage relies heavily on compound quality and purity. Researchers working with peptide candidates typically source laboratory-grade peptide compounds that meet strict analytical standards, since even minor impurities can produce misleading results during early screening.
Stage 2: Preclinical Research
Once a lead compound is identified, it enters preclinical research—a phase designed to evaluate safety and biological activity before any human testing begins.
Preclinical studies involve two main components. In vitro testing examines how the compound behaves in cell cultures and tissue samples. In vivo testing evaluates its effects in animal models, typically rodents, to assess toxicity, dosing ranges, absorption, distribution, metabolism, and excretion (collectively known as ADME).
Regulatory agencies like the FDA require preclinical data to demonstrate that a drug is reasonably safe before approving an Investigational New Drug (IND) application, which grants permission to begin human trials.
For peptide candidates, preclinical research also involves evaluating compound stability under various conditions—since peptides are sensitive to temperature, light, and moisture. Researchers must ensure that the compound they’re testing in animal models is the same compound they characterized in discovery, which is why sourcing from certified research peptide suppliers with documented Certificates of Analysis matters at this stage.
Preclinical testing typically takes 1 to 3 years. Only compounds with strong safety profiles and clear biological activity advance to the next phase.
Stage 3: Clinical Trials
Clinical trials are the most visible, expensive, and time-consuming part of the drug development process. They’re conducted in three sequential phases, each with a distinct purpose.
Phase I: Safety and Dosing
Phase I trials enroll a small group of healthy volunteers (typically 20–100) to evaluate safety, determine safe dosage ranges, and identify side effects. The primary goal isn’t to test whether the drug works—it’s to confirm it doesn’t cause unacceptable harm at therapeutic doses.
Phase II: Efficacy and Side Effects
Phase II expands testing to several hundred patients who actually have the target condition. Researchers assess whether the drug produces its intended effect, refine dosing, and monitor for adverse reactions. This is where many drug candidates fail—roughly 70% of compounds that pass Phase I don’t survive Phase II.
Phase III: Large-Scale Confirmation
Phase III trials involve thousands of patients across multiple sites, often internationally. These studies compare the new drug against existing treatments or placebos using randomized, controlled designs. The data generated here forms the backbone of the regulatory submission.
Peptide-based therapies have shown strong performance in recent Phase III programs. GLP-1 receptor agonists, originally studied for diabetes, demonstrated significant results in large-scale weight management trials—findings that have driven substantial interest in peptide-based weight management research and accelerated investment across the biotech sector.
The entire clinical trial process typically spans 6 to 10 years, though expedited pathways exist for compounds addressing serious unmet medical needs.
Stage 4: FDA Review and Approval
After successful Phase III trials, the drug developer submits a New Drug Application (NDA) or Biologics License Application (BLA) to the FDA. This submission includes all preclinical and clinical data, manufacturing details, proposed labeling, and the complete safety profile.
The FDA review process involves several steps. A team of scientists, physicians, and statisticians evaluates the evidence. An advisory committee of independent experts may weigh in on whether the benefits outweigh the risks. The FDA then makes one of three decisions: approve the drug, request additional data, or deny the application.
Standard review takes approximately 10 months. Priority review—reserved for drugs that offer significant improvement over existing options—shortens this to about 6 months. Breakthrough therapy designation and accelerated approval pathways can compress timelines further for critical treatments.
The FDA drug approval process is rigorous by design. Only about 1 in 10 compounds that enter clinical trials ultimately receive approval, which is why the earlier stages of development focus so heavily on filtering out weak candidates early.
Stage 5: Post-Market Monitoring (Phase IV)
FDA approval isn’t the end of the drug development process—it’s the beginning of long-term surveillance.
Phase IV studies, also called post-market monitoring, track the drug’s performance in real-world conditions across much larger and more diverse patient populations than clinical trials allow. These studies can reveal rare side effects, long-term risks, or new therapeutic applications that weren’t apparent during controlled trials.
The FDA can require label changes, issue safety warnings, or even withdraw a drug from the market if post-approval data reveals significant concerns. Manufacturers are also required to report adverse events and maintain manufacturing quality standards throughout the drug’s commercial life.
This ongoing oversight is especially relevant for newer drug classes. As peptide-based therapies continue reaching the market, post-approval research helps build the long-term safety evidence that prescribers and patients need to make informed decisions.
Why Peptide-Based Therapies Are Reshaping the Pipeline
Peptides occupy a unique position in the drug development landscape. They’re smaller than traditional biologic drugs (like antibodies) but more targeted than most small-molecule drugs. This gives them several advantages in the development pipeline:
Faster synthesis and optimization. Peptides can be synthesized and modified more quickly than large proteins, shortening the discovery phase.
Higher target specificity. Their structure allows precise receptor binding, which can reduce off-target effects and improve safety profiles during clinical trials.
Growing regulatory familiarity. As more peptide-based drugs reach the market successfully, regulatory pathways become better established, reducing uncertainty for developers.
Expanding therapeutic areas. Beyond metabolic conditions, peptide candidates are being studied for tissue repair, neuroprotection, antimicrobial applications, and oncology—broadening the pipeline significantly.
These advantages help explain why peptide drug development has accelerated in recent years, with dozens of candidates currently in various phases of clinical trials worldwide.
Frequently Asked Questions
What are the 5 stages of drug development? The five stages are discovery and development, preclinical research, clinical trials (Phases I–III), FDA review and approval, and post-market safety monitoring. Each stage builds on the previous one, progressively confirming safety and efficacy before a drug reaches patients.
How long does the drug development process take? On average, 10 to 15 years from initial discovery to market approval. Clinical trials alone typically span 6 to 10 years, though expedited regulatory pathways can shorten timelines for drugs addressing serious conditions.
What are the 4 phases of drug development? The four clinical phases are Phase I (safety/dosing in healthy volunteers), Phase II (efficacy in patients), Phase III (large-scale confirmation), and Phase IV (post-market monitoring). These sit within the broader five-stage development framework.
What is drug development called? The overall process is commonly referred to as the drug development pipeline or pharmaceutical development process. The early research portion is often called drug discovery, while later stages are referred to as clinical development.
What are the 5R’s of drug development? The 5R framework—Right target, Right tissue, Right safety, Right patient, Right commercial potential—is a strategic model developed by AstraZeneca to improve success rates in drug development by ensuring key criteria are met before advancing a compound through each stage.
Final Thoughts
The drug development process is long, expensive, and deliberately rigorous—because the stakes are high. Every stage exists to answer a specific question about whether a compound is safe, effective, and worth bringing to patients.
What’s changing is the type of compounds moving through this pipeline. Peptide-based therapies are demonstrating that smaller, more targeted molecules can navigate the development process efficiently and deliver meaningful clinical results. As this field continues to mature, the fundamental stages of drug development remain the same—but the candidates filling them are evolving rapidly.
References
- DiMasi, J. A., Grabowski, H. G., & Hansen, R. W. (2016). Innovation in the pharmaceutical industry: New estimates of R&D costs. Journal of Health Economics, 47, 20–33.
- Fosgerau, K., & Hoffmann, T. (2015). Peptide therapeutics: Current status and future directions. Drug Discovery Today, 20(1), 122–128.
- U.S. Food and Drug Administration. (2025). The Drug Development Process. FDA.gov.
- Muttenthaler, M., et al. (2021). Trends in peptide drug discovery. Nature Reviews Drug Discovery, 20(4), 309–325.
- Henninot, A., Collins, J. C., & Nuss, J. M. (2018). The current state of peptide drug discovery. Journal of Medicinal Chemistry, 61(4), 1382–1414.