Cancer, a universal malady, presents a double-edged sword in its complexity and the significant financial burden of its treatment. Representing a multitude of diseases with different pathologies, every cancer variant demands a specialized treatment strategy, thus escalating costs and making it unattainable for many. In this daunting scenario, CytoMed Therapeutics, under the expert guidance of Chief Operating Officer, Dr. Wee Kiat Tan, brings forth a disruptive solution – the ‘one product, many patients’ approach. This innovative methodology turns the tables on traditional treatment paradigms, promising a radical shift in how cancer is combatted. A unique blend of cellular science and cutting-edge biotechnology, this strategy offers a versatile, single product with the potential to treat a wide array of cancer types. More importantly, this approach paves the way for a more equitable healthcare landscape, underlining the importance of affordability in the complex world of cancer treatment. By addressing the prohibitive costs traditionally associated with cancer therapy, CytoMed has positioned itself as a catalyst for change, hinting at a future where high-quality cancer treatment is both universally effective and financially accessible.
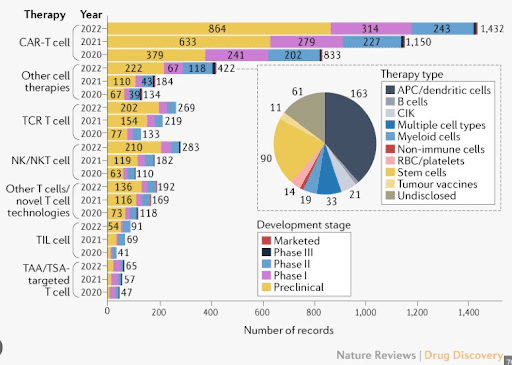
Chimeric Antigen Receptor T-cell (CAR-T) technology has been a milestone in cancer therapeutics, providing a beacon of hope for patients battling this arduous disease. Traditional CAR-T cell therapy involves genetically modifying a patient’s own T cells to recognize and fight cancer cells, essentially weaponizing the immune system. Despite the breakthroughs this technology has provided, it carries notable limitations. The high cost associated with individualized treatment, coupled with a complex logistical process involving extraction, modification, and re-infusion of cells, has restricted its accessibility. Furthermore, the patient-specific nature of this therapy raises scalability issues and prolongs waiting times.
Enter CytoMed Therapeutics, which aims to transcend these challenges with its novel CAR-T cell technology. Utilizing Gamma Delta T cells from healthy donors, CytoMed’s approach has the potential to serve a multitude of patients without the need for customization. Gamma Delta T cells, an underappreciated subset of the immune system, exhibit potent anti-cancer capabilities. Their inherent property to recognize a wide range of cancers without the necessity for major histocompatibility complex recognition offers a significant advantage, making them universally applicable.
This broad applicability promises a sea change in manufacturing costs and patient waiting times. By enabling an ‘off-the-shelf’ product, CytoMed not only democratizes access to cutting-edge cancer therapies but also reduces logistical complexity. This innovative approach sets the stage for large-scale production, leading to a significant reduction in costs per treatment and an expedited process from diagnosis to treatment. As we strive for a more inclusive healthcare future, it’s clear that CytoMed’s novel approach to CAR-T cell technology is playing a vital role in reshaping cancer therapy.
A significant barrier in the ongoing battle against cancer is the prohibitive cost of treatment, with CAR-T therapy being a prime example. The current model of CAR-T therapy, while effective, presents financial hurdles that often put it out of reach for many patients. This is largely due to the individualized nature of the treatment – the process of extracting a patient’s T cells, genetically modifying them, and re-infusing them back into the patient is a logistically complex and costly endeavor.
However, CytoMed Therapeutics’ innovative ‘one product, many patients’ approach promises to address these affordability issues. The utilization of universally applicable Gamma Delta T cells from healthy donors allows the company to manufacture a single ‘off-the-shelf’ product capable of treating a wide range of cancer types. This large-scale production approach reduces the per-patient cost significantly compared to the traditional CAR-T therapy. With this revolutionary shift in methodology, the ‘one product, many patients’ concept could be the key to democratizing access to advanced cancer treatments. “You could have the best solution to a disease or treatment, but if it is not affordable, how are you going to use it? A lung transplant, for instance, costs two million US dollars. So, you have a good solution but you have to make it affordable,” emphasizes Dr. Tan.
Further contributing to cost reduction is CytoMed’s strategic location in Southeast Asia. Known for its competitive cost structure, the region provides economic advantages that are reflected in CytoMed’s pricing strategy. Labor costs, operational expenses, and even certain regulatory costs in Southeast Asia are generally lower compared to Western countries. These cost savings, coupled with the mass production capabilities of the ‘one product, many patients’ approach, help CytoMed significantly lower the cost of its CAR-T therapy, making it a more accessible option for cancer patients globally.

“We believe in delivering treatment that is accessible to all, not just a privileged few. Our ‘one product, many patients’ approach is a stepping stone towards achieving this goal,” states Dr Tan, COO of CytoMed. By focusing on these strategic aspects, CytoMed is leading the charge towards an era of more affordable, accessible, and efficient cancer treatment, reaffirming that the fight against cancer is not a privilege, but a universal right.
The technological innovations of CytoMed Therapeutics could herald a new chapter in cancer treatment, redefining accessibility and affordability in CAR-T therapy. By engineering a universally applicable product, CytoMed can dramatically expand the reach of CAR-T therapy, extending its benefits to a larger population segment that was previously underserved due to financial constraints.
The potential impact of this approach on patient outcomes is profound. By making effective CAR-T therapy more readily available, the ‘one product, many patients’ model could improve survival rates and enhance the quality of life for countless cancer patients. The implications for healthcare systems and global health are equally momentous. Affordable and universally applicable CAR-T therapy would relieve substantial financial pressure from healthcare systems worldwide, redistributing resources to other vital areas.
Looking to the future, CytoMed’s relentless drive for innovation continues to push the boundaries of what’s possible in CAR-T therapy. Further research and advancements in the field, such as improving the efficiency of Gamma Delta T cell extraction and refining their precision targeting capabilities, are underway. By continually optimizing their ‘one product, many patients’ approach, CytoMed is paving the way towards a future where high-quality cancer therapy is not a luxury, but a standard accessible to all.
CytoMed Therapeutics recently received approval to commence a pivotal Phase One clinical trial in Singapore, marking a significant milestone in its ‘one product, many patients’ journey. This trial presents a unique opportunity to test the efficacy and safety of their universally applicable CAR-T therapy in a real-world setting. The results could substantiate the potential of this innovative approach, furthering its acceptance within the medical community. Moreover, a successful trial outcome may serve as a practical demonstration of the cost-effectiveness of their model, emphasizing how cutting-edge cancer therapy can be made both effective and affordable for a diverse patient population.

CytoMed’s ‘one product, many patients’ strategy represents a potential game-changer in the field of cancer therapy. By transforming a traditionally complex and high-cost treatment into an affordable and universally applicable solution, it has potential to fundamentally reshape the landscape of cancer care. The importance of affordability in cancer treatment can’t be overstated, and CytoMed’s pioneering approach seeks to address this critical issue. As we look towards the future of cancer therapy, it’s encouraging to see innovative companies like CytoMed relentlessly pushing the boundaries of what’s possible. Their ongoing research and advancement efforts serve as a beacon of hope for millions of patients worldwide.