Although analysts keep touting how the use of AI across the pharmaceutical industry will continue to grow, the industry’s ability to treat disease still has a long way to go. We often read headlines about groundbreaking biotechnology advancements in the news, but the fine print says that these innovations may take a decade or more to reach the market. Often, the promised advances never materialize, and now AI drug discovery has shown similar dismal failure rates to conventional drug discovery – around 90%. So far, we can treat only about 500 out of the 10,000 known human diseases, and many of those treatments are far from perfect. “Clearly, there is a pressing need for a faster and better way to bring breakthrough treatments to patients,” says Adityo Prakash, CEO of drug-discovery company Verseon.
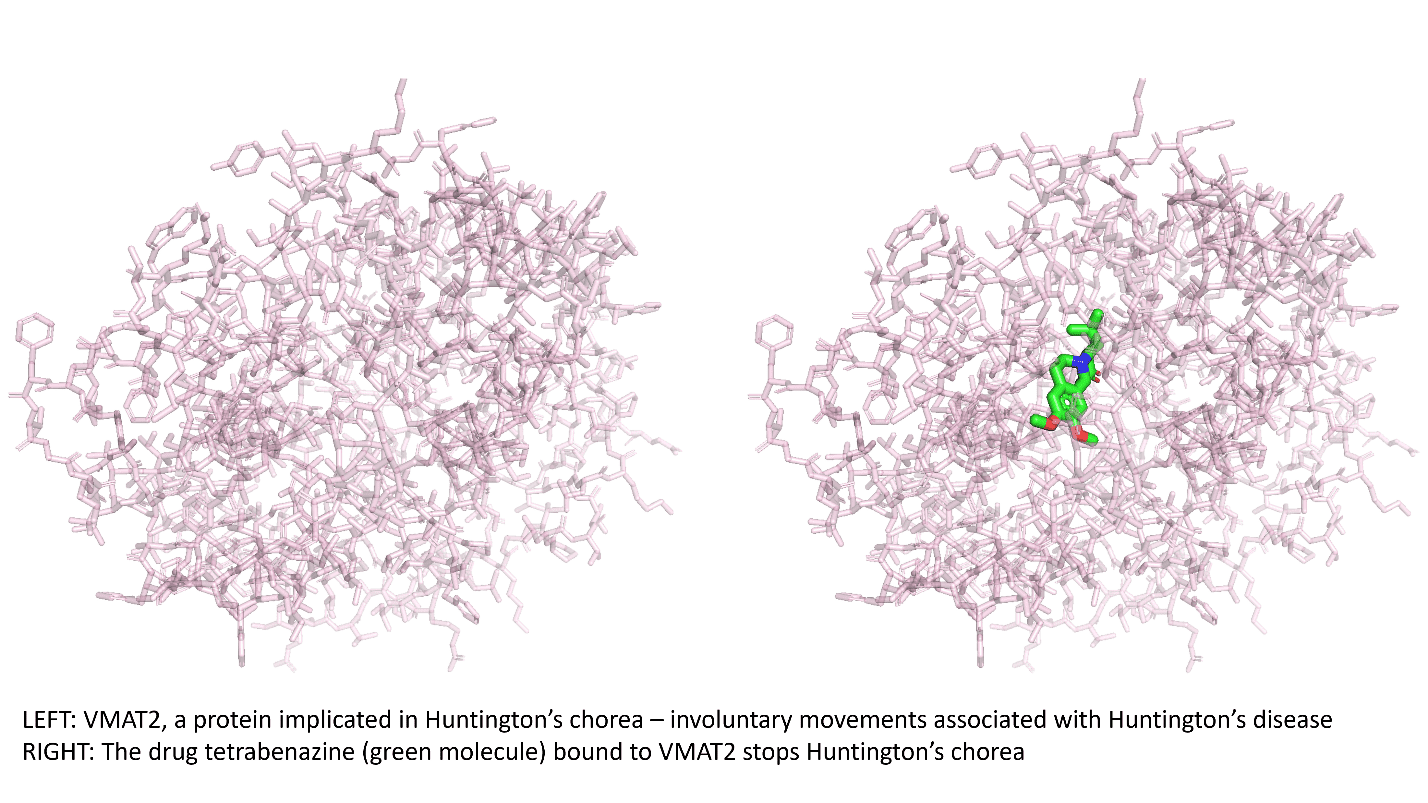
To fully grasp the challenges in finding new drugs, it is important to understand how the proteins in our bodies contribute to disease. While most cell structures in living organisms are made of various proteins, some of these proteins are also involved in disease processes. The objective of drug discovery is to find molecules that are binders to the target proteins involved in disease processes and stop their disease-causing behavior. More often than not, these drugs are small molecules that can be taken orally, absorbed through the gut into the bloodstream, and enter cells readily. Once a drug molecule binds to a target, it alters the target protein’s function and stops the associated disease from progressing.
Biologists estimate that there are approximately 11,000 different proteins in the human body that are involved in various physiological processes with potential relevance to diseases. Those proteins comprise what is called the “druggable human proteome.” But at this point, the pharmaceutical industry and academia have identified binders for fewer than 900 of these proteins, and many of those binders will never make suitable drugs because they exhibit toxicity or other undesirable properties. The slow progress is rooted in the drug discovery and development process itself.
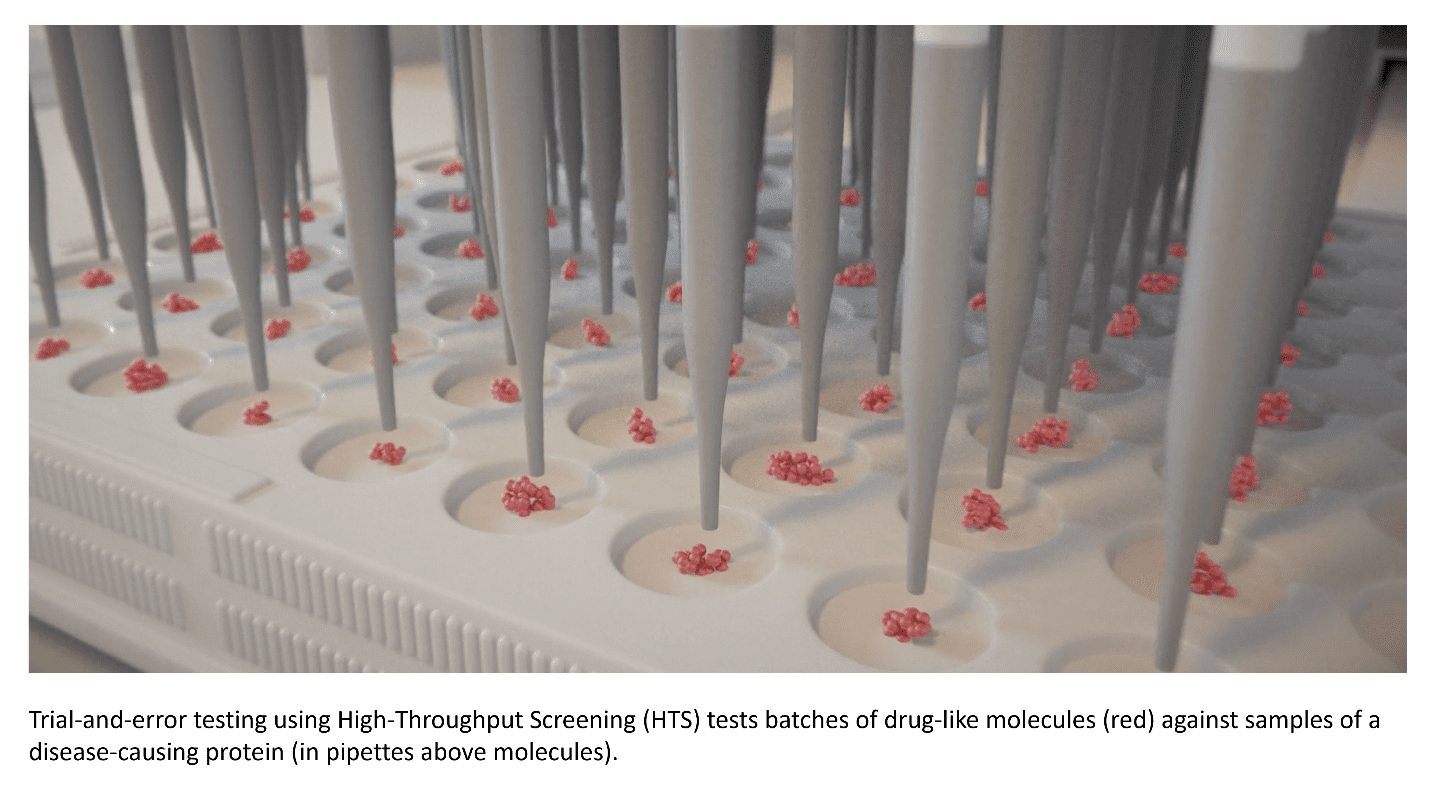
Traditionally, the search for new drugs has relied on testing large numbers of molecules in the lab to see if any of them have useful effects. While this trial-and-error approach has delivered important therapies, it is slow, expensive, and uncertain. The odds of success are low, and most programs never result in a treatment that reaches patients.
In recent years, AI has proven valuable in some areas of healthcare, but when it comes to discovering entirely new types of medicines, it faces real limits. AI can only work with the data it has been trained on, data which covers an insignificantly miniscule fraction of all possible drug-like molecules – a mere 100 million (10^8) out of a novemdecillion (10^60) possibilities. This fact means AI tools typically suggest variations of compounds already explored, rather than identifying truly new drug candidates. If healthcare is to address long unmet needs, researchers must find new tools that allow them to go beyond trial and error and explore drug-like molecules that would otherwise remain out of reach.
To break free from the limitations of current drug discovery, the pharmaceutical industry needs a systematic way to design new molecules that have never been made before and yet have the potential to be safe, effective, potent binders to disease-causing proteins. This process can be likened to designing new buildings using CAD/CAM tools, which help architects create completely new designs that meet structural-stability and functional requirements.
Verseon, a Silicon Valley company, has built an atomic-level analog to the CAD/CAM tools used in structural design that enables the company to systematically design novel drug molecules. For any protein structure, Verseon’s platform uses its breakthroughs in quantum physics modeling to design multiple distinct new binders. To select the best candidates for clinical trials, the company makes each of the identified novel binders and subjects them to rigorous chemical and biological testing in the lab. Verseon’s new AI models train on this proprietary lab data to suggest further variants, which are in turn subjected to more tests before advancing to clinical trials.
Verseon is not the first company to attempt harnessing physics-driven modeling for drug discovery. Starting in the late 20th century, New York-based scientific software and biotechnology company Schrödinger and UK nonprofit Cambridge Crystallographic Data Centre (CCDC) have built and sold physics-based design solutions for drug discovery. Most pharmaceutical and biotech companies have used tools from these companies for the past three decades, though the industry’s low and declining R&D productivity suggests that these commercially available tools in their current state are unable to reliably drive design of new drug molecules. Pfizer’s former Senior Vice President of R&D Strategy Robert W. Karr says, “Licensing computational drug-discovery tools instead of using them exclusively for in-house drug development is a good indicator that the tools don’t have the necessary predictive accuracy. Current commercially available tools lack the ability to replace trial-and-error testing to design completely new drugs for which there are no prior experimental data.” So, pharmaceutical companies are stuck relying on slow, expensive and unpredictable experimental methods for drug discovery, while commercial computational modeling tools are relegated to ancillary visualization and decision support roles.
Integrating the right advances in physics, chemistry, and biology with AI as Verseon has done has the potential to entirely replace the current trial-and-error paradigm, offering far more efficient and targeted drug discovery. Intellia CEO and former AbbVie CSO John Leonard, who has tested Verseon’s technology, says, “Verseon’s revolutionary platform shifts the discovery paradigm from handcrafted techniques into a systematic industrial process.”
Using their platform, the company has focused on in-house drug discovery and built a growing pipeline of 16 drug candidates for preventing heart attacks and strokes, stopping vision loss from diabetes, treating cancers, and more. The patient populations for these conditions are large, often numbering in the hundreds of millions for a single drug candidate. “Any one of these drugs reaching market would create a large pharma company almost overnight. But unlike the typical ‘one-drug wonder’ biopharma company, Verseon has built a scalable drug-discovery process producing a stream of novel drug candidates that would change what medicine can do for humanity,” says Richard Serbin, former Vice President of Corporate Development at Johnson & Johnson.
As medicine manages to scale up the process of systematically finding drugs for disease-causing proteins, it will gain the ability to precisely target and control both chronic and acute diseases – and reduce strain on healthcare systems worldwide. “Being able to address an increasingly large part of the druggable proteome will allow us to maintain the human body in a state of optimal function for much longer than is possible today,” says Verseon CEO Adityo Prakash.
Verseon’s results suggest that the future of drug discovery will not be governed by rare moonshots, but rather by a sustainable, systematic process. For patients and investors alike, pursuing scalable drug discovery and development is more than an innovative business strategy. It presents the possibility of a future in which transformative treatments for disease are the norm, not the exception.