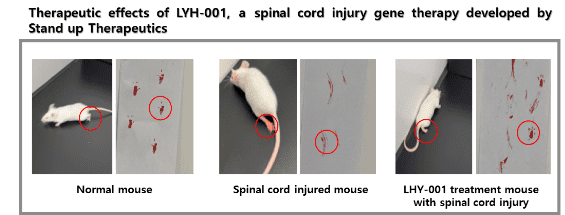
Stand Up Therapeutics Co., Ltd. is a gene therapy company in South Korea that helps paralyzed people with spinal cord damage. Stand Up Therapeutics said that it had signed a contract with VectorBuilder Inc., a global leader in gene delivery technologies, to make a GMP-grade gene delivery system.
Vector Builder is the biggest company in the world that makes custom vectors for delivering viral and non-viral genes. It is a multinational company with offices in North America, Europe, China, Japan, Korea, Australia, and Israel. The company’s main office is in Chicago, Illinois, and it also has offices in North America, Europe, China, Japan, Korea, Australia, and Israel. At the moment, more than 80,000 custom vectors are made for scholars all over the world every year. Vector Builder has GMP facilities for gene delivery systems and sells gene therapy products and delivery systems to over 50,000 customers and tens of thousands of institutions, such as the top pharmaceutical companies and universities in the world. We sell items and ways to deliver gene therapy.
Stand Up Therapeutics says that under this deal, Vector Builder will make and sell gene therapy products that Stand Up Therapeutics has designed in the future. The goal is to take control of the world market.
Bruce Lahn, the chief scientist of VectorBuilder, said, “VectorBuilder is interested in Stand Up Therapeutics’ gene therapy for treating paralysis all over the world. As a world leader in designing and making GMP gene delivery vectors, I hope that VectorBuilder will be able to sell this product to Stand Up for Patients around the world.”
Dr. Junsang Yoo, the CEO of Stand Up Therapeutics, said, “With its direct lineage reprogramming technique, Stand Up Therapeutics is the only company in the world with the technology to treat paralyzed patients with damaged spinal cords” (STUP-001). Members of Stand Up Therapeutics are putting all of their time and energy into making a treatment for people who are paralyzed because of a terrible accident. Also, “Stand Up Therapeutics hopes to use the direct cross-differentiation PIPELINE technology to make treatments for Parkinson’s disease (STUP-002), stroke (STUP-003), spinal stenosis (STUP-004), and myocardial infarction (STUP-005) in the future.”
In the first quarter of 2023, four paraplegic patients will take part in IIT Clinical phase I/IIa research on STUP-001, a gene therapy treatment for spinal cord injury (SCI).
When talking about Stand Up Therapeutics Co., Ltd.
Stand Up Therapeutics Co., Ltd. creates gene therapies for nerve cell regeneration by turning fibroblasts into motor neurons in vivo. These therapies use cutting-edge technologies. Stand Up Therapeutics plans to use direct lineage reprogramming technology to treat patients with mild and severe paralysis. These patients, who make up about 1.9% of the world’s population and have spinal cord and brain injuries, will be treated with direct lineage reprogramming. Stand Up Therapeutics will add more drugs to its pipeline by making direct reprogramming and gene therapy safer and more effective.
You can find more information at http://stutps.com.